ISPOR Europe 2023
12 - 15 November
Copenhagen, Denmark
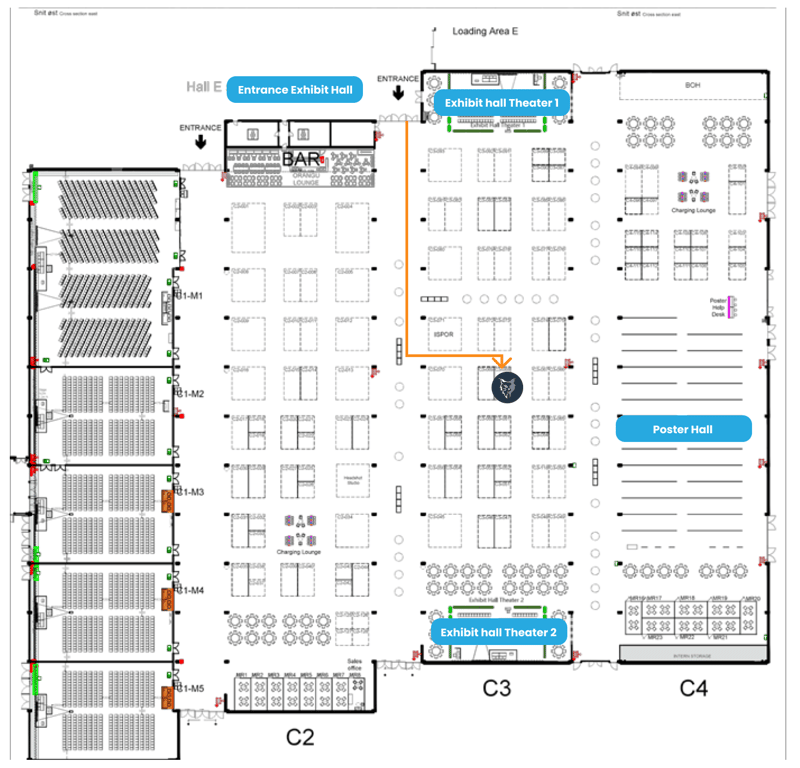
Booth C3-068
Whether you are a Market Access, RWE, HEOR, or Medical Affairs professional, LynxCare can give you access to rich insights gained from highly granular and qualitative fresh real-world data to answer your research questions throughout the drug development life cycle.
We invite you to visit our booth and explore the possibilities the LynxCare solution has to offer firsthand. Learn how you can receive those deep insights that are needed to push your research studies forward, and with the level of quality necessary to improve patients’ lives.
What to expect
We will be showcasing our real-life use cases, and will demonstrate how, together with our partners, we have positively impacted patient outcomes. Our RWE-experts team will be available to answer your questions, and discuss potential collaborations.
Set up a meeting through the calendar on the right for an informal talk, so we can listen to your needs and discuss where LynxCare can be of help. 👉
We look forward to seeing you at the Bella Center in Copenhagen!
Poster presentation
Building Federated Data Networks With Common Data Models to Generate Insights Through Real-World Evidence Observational Studies in Oncology
📅 Date: 14 November
🕔 Time: 12.30 - 13.30
Read our abstract here or continue reading below 👇.
Meet
Georges De Feu (CEO, co-founder), Stephan Engelen (Chief Commercial Officer), Shahbaz Pervaiz (RWE Services Director), Carlos Bringas (Sales RWE Manager), Clara L. Oeste (Senior Medical Writer).
Choose a timeslot in the calendar on the right to speak to one of our RWE experts present at our booth.
More about LynxCare
Unlocking clinical insights from hospital care
LynxCare is a health tech company at the forefront of the healthcare data revolution. By harnessing the power of innovative technology (AI + NLP), we unlock all valuable data within hospitals (incl. narrative data gathered in medical records), build harmonized clinical databases, empowering hospitals and life sciences organizations to leverage real-world evidence for groundbreaking research and improving patient care.
Where to find us
Check the ISPOR Exhibitor resources for the latest updates on the floor plan:
https://www.ispor.org/conferences-education/conferences/upcoming-conferences/ispor-europe-2023/exhibits-sponsorship/exhibitor-resources
Building Federated Data Networks With Common Data Models to Generate Insights Through Real-World Evidence Observational Studies in Oncology
AUTHOR(S): Oeste C [1], Hens D [1], Fovel I [2], Bringas C [1], Encarnacion E [1], De Feu G [1]
[1] LynxCare, Leuven, Flemish Brabant, Belgium, [2] LynxCare, Everberg, Belgium
OBJECTIVES
Accessing and standardizing raw clinical data across multiple hospitals presents a challenge in Oncology. However, it is crucial to use real-world data sources such as electronic health records (EHR) to leverage untapped information. We are building a federated data network to facilitate GDPR-compliant data exchange of large datasets, with hospitals as owners. This network, governed by a common data model (CDM), is aimed at fostering multicenter, observational, real-world evidence (RWE) studies in Oncology, with breast cancer, lung cancer, and immunotherapy as therapeutic areas of focus.
METHODS
Oncology studies are ongoing in participating Belgian hospitals using the LynxCare data processing technology over 4 different EHR systems to automatically process structured and unstructured data, using natural language processing for the latter, to generate OMOP-CDM databases. Variables (n = 1056) include demographics, comorbidities, cancer diagnosis, tumor staging, performance status, oncology treatments and procedures (including immune checkpoint inhibitors), anatomical pathology data (genomics), and adverse events. Collaborating with key opinion leaders (KOLs), we established an Oncology research group in Belgium that is redefining data dictionaries, establishing a shared comprehension per variable and enhancing data granularity.
RESULTS
To date, the Oncology data network comprises 71.572 patients, for which more than 5 million unstructured records were processed and there were 27149 mappings from structured data sources (e.g., administered and prescribed medication, laboratory parameters, multidisciplinary Oncology consult, and mortality). More than 1000 quality-controlled variables are measured continuously to identify patterns, trends, and associations between datapoints and patient outcomes.
CONCLUSIONS
Clinicians can leverage the resulting RWE to develop personalized treatment plans based on patients’ specific characteristics, disease progression, and prognostic factors. These insights can also inform evidence-based guidelines and regulatory decisions. This groundbreaking initiative will expand to other European countries, providing a sandbox of federated data networks for multicenter RWE studies in Oncology, but also paving the way towards precision medicine.
CONFERENCE/VALUE IN HEALTH INFO
2023-11, ISPOR Europe 2023, Copenhagen, Denmark
Value in Health, Volume 26, Issue 11, S2 (December 2023)
CODE
RWD84
TOPIC
Clinical Outcomes, Methodological & Statistical Research, Real World Data & Information Systems, Study Approaches
TOPIC SUBCATEGORY
Artificial Intelligence, Machine Learning, Predictive Analytics, Clinical Outcomes Assessment, Distributed Data & Research Networks, Electronic Medical & Health Records
DISEASE
Drugs, Oncology, Personalized & Precision Medicine
Source: https://www.ispor.org/heor-resources/presentations-database/presentation/euro2023-3786/133413
